Applied Cell Therapy: Turning Immune Cells into Childhood Brain Cancer Fighters
A Deadly Problem
Every day in the United States, nearly a dozen children are diagnosed with brain and other central nervous system (CNS) tumors. These comprise the third most common type of childhood cancer, behind leukemia and lymphoma, and the deadliest form. Of the approximately 4,200 children diagnosed with these lethal tumors (of which approximately 2,000 are brain cancers) each year, fewer than 20 percent will survive. This is despite decades of research conducted at medical centers across the United States.
Even those who do survive often face recurrence as well as serious long-term effects that can include cognitive deficits, neuropathy, hearing loss and other conditions stemming from both their disease and the impact of treatment on developing brains. Thus for many, survivorship can mean physical impairment, learning challenges and emotional trials. Clearly, there is a need for developing more effective, less toxic therapies that will increase the survival of children with brain cancer while also assuring them a higher quality of life.
Providing renewed hope for children with brain cancer requires a new way of thinking. Unfortunately, pediatric cancer researchers have reached a point of diminishing returns using the current approach which relies on for-profit industrial concerns to develop and test new treatments. This is because biopharmaceutical companies, perceiving inadequate return on financial investments in research for so-called “orphan” diseases with small patient populations, have little incentive to investigate new therapies for pediatric brain tumors. However, industry can provide pediatric oncologists—to whom falls the challenge of developing and implementing new treatments for these cancers—with a model that harnesses metrics and milestones to implement new therapies within academia.
A Promising Solution
Laurence J. N. Cooper, M.D., Ph.D., head of the Pediatric Bone Marrow Transplantation (Cell Therapy) Section at The University of Texas MD Anderson Children’s Cancer Hospital, embraces the challenge to develop and implement new approaches to treating brain cancers. His priority has long been to develop new, effective and safe therapies for children, adolescents and young adults with cancer. And his Pediatric Cell Therapy Program is successfully translating basic science discoveries into new targeted therapies that use the body’s immune system to attack childhood cancers.
Dr. Cooper’s novel approach is based on training immune cells, specifically natural killer (NK) cells and T cells, to seek and destroy cancer cells. His research has resulted in multiple papers and patents that cover such innovations as the design of new receptor molecules that redirect T cells to attack invading tumor cells. Now being tested in first-in-human clinical trials available only at MD Anderson, these immune-based therapies already are offering new hope for treating leukemias and lymphomas. Dr. Cooper wants to extend application of this promising, personalized therapy approach to pediatric brain cancers, and he seeks philanthropic support toward this critical goal. These monies will enable his team, under the auspices of the U.S. Food & Drug Administration, to implement a trial to infuse NK cells into patients with medulloblastoma—and to develop next-generation approaches for future clinical trials.
Dr. Cooper proposes to continue his assault on pediatric brain cancers with a focus on medulloblastoma as a research and treatment model. Each year, this brain cancer sub-type is diagnosed in approximately 500 U.S. children, 40 percent of whom are under the age of five. According to the America Society of Oncologists, the overall survival rate for medulloblastoma patients younger than 15 is 62 percent. And this rate declines significantly when the cancer recurs. As Dr. Cooper works to develop and implement new therapies against medulloblastoma, he plans to apply what he learns against other types of brain cancer.
A Foundation of Expertise, the Right Environment for Discovery
Dr. Cooper holds a joint appointment in MD Anderson’s Department of Immunology and collaborates with a multitude of investigators—in melanoma, lymphoma, neuro-oncology and other areas—who share his research focus and passion for the therapeutic potential of NK-cell and T-cell therapies. Operating like a biomedical company, but on a not-for-profit basis within the superstructure of MD Anderson, his laboratory has launched a pipeline of cellular biologics for children and adults with advanced cancers. The technologies Dr. Cooper has developed make possible the propagation of large numbers of human immune cells that are suitable for human application, enabling current and future clinical trials to infuse these cells into patients.
This process begins with harvesting the patient’s own NK cells or T cells, growing their numbers in the laboratory, and engineering them to zero in on specific tumor targets while sparing normal cells. These modified, cancer-killing immune cells are then reinfused into the patient in an approach that may be lifesaving for those whose cancers have progressed despite their receiving conventional chemotherapy, radiation and surgery.
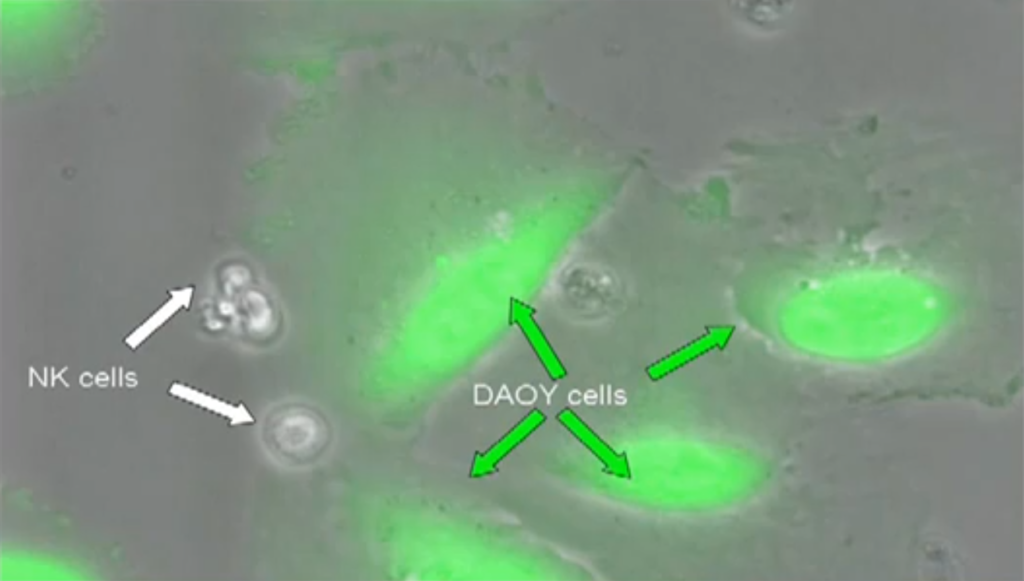
Dr. Cooper’s unique clinical method hijacks the innate power of NK and T cells to kill tumor cells while clearing a significant hurdle: even though a patient’s immune cells can kill, they initially cannot recognize tumor cells. After all, most patients who develop cancer do so with a normal immune system in place. To counteract the ability of tumor cells to hide from immune cells, Dr. Cooper adapts NK cells and T cells in the laboratory so that they find and dock to tumor cells. This engages the immune cells’ natural killing ability, leading them to wipe out the tumor cells to which they bind (see movie).
A Critical Need
While the survival rates for some childhood cancers have improved (overall mortality rates have declined 53 percent since 1975), little to no progress has been made against pediatric brain tumors. This sad scenario seems unlikely to change any time soon given the relative rarity of childhood brain cancers and, as noted previously, the unwillingness of for-profit biomedical and pharmaceutical companies to devote considerable research and development resources to therapies that will ultimately impact children with cancer. Support from traditional funding mechanisms is limited as well. The National Cancer Institute, for example, invested only $157 million in research for all brain and other CNS cancers collectively (both pediatric and adult) in FY2010—or only about 3 percent of its $5.12 billion budget that year. Given that today’s estimated cost of developing any new therapy and bringing it to market can well exceed $500 million over 10 years or longer, this level of research investment falls far short. Philanthropic support is urgently need to fill the gap.
TARGETING MEDULLOBLASTOMA: A FOUNDATION FOR MOVING FORWARD
Relapsed medulloblastoma is difficult to treat and nearly impossible to cure. New therapies are mandated since conventional treatments based on chemotherapy, radiation, and surgery are ineffective against advanced disease and furthermore lead to toxicity. Translating immunology into immunotherapy to answer this need, Dr. Cooper has developed NK cells as a new investigational treatment for advanced medulloblastoma. His groundbreaking work was prompted by an appeal from the family of Noah, a 8-year-old patient diagnosed with this aggressive cancer. Answering the family’s request for “out of the box” thinking, Dr. Cooper and his team embarked on the “Noah Protocol,” a program to improve the therapeutic potential of NK cells. His approach is based not only on manufacturing large numbers of cells, but also on increasing their potency—a critical combination given that a patient’s circulating NK cells normally number only a few, and these reside in a dormant state. Dr. Cooper achieved these objectives by using cellular engineering to generate a “nurse” cell line of artificial antigen-presenting cells, or aAPCs. These nurse cells were genetically modified to express molecules that could activate NK cells for proliferation and for tumor killing.
The generation of aAPCs enabled development of culture systems to numerically expand NK cells for human application. This is a major advance for the immunotherapy field as it was not previously possible to generate NK cells in the quantity and quality needed for therapy. Dr. Cooper’s program has undertaken the cost to produce these cells in MD Anderson’s manufacturing suites so they can be used to generate clinical-grade NK cells—thus providing the right infrastructure required to launch a clinical trial for multiple patients, such as Noah, who might benefit from infusions of NK cells to treat their relapsed medulloblastoma.
Further innovation will advance this effort as Dr. Cooper and colleagues have found a way to overcome unique challenges which complicate new therapy development for cancers of the brain. The success of employing NK cells against brain tumors such as medulloblastoma will hinge on their ability to cross the brain’s natural shield—the blood-brain barrier (BBB) that protects it against foreign substances and immune cells. Brain tumors co-opt this “wall,” using it to hide from cells of the immune system. To win this deadly game of hide-and-seek, Dr. Cooper has collaborated with neurosurgeons to strategically place catheters which exit near a tumor. These catheters serve as a conduit by which NK cells can be infused to bypass the BBB and gain access to the tumor microenvironment. There, these immune cells can go to work, programmed as they are to attack only tumor cells while leaving healthy cells alone. In his proposed clinical trial, Dr. Cooper seeks to evaluate the ability of propagated NK cells to target advanced medulloblastoma—with the expectation of curing this disease.
A TOOL FOR FIGHTING OTHER BRAIN CANCERS
The development of clinical-grade NK cells for investigational therapy in children with advanced medulloblastoma has potential impact well beyond this single type of cancer. Using the same platform technologies created for medulloblastoma, Dr. Cooper plans to expand this immunotherapy into comprehensive treatment approaches against other pediatric malignant brain tumors. This future hit list includes diffuse interstitial pontine glioma (DIPG) which, like so many brain cancers, is woefully understudied and remains virtually untreatable despite the best research efforts so far. Dr. Cooper’s vision can be readily implemented since data accumulated to date already demonstrates proof-of-principal using laboratory-based science. He and his team have developed a model for brain cancer initiatives based on achieving the following milestones, along a timeline allowing for inspection of progress and dictated by available funding.
1. Validate the processes to generate immune cells for human application. This will include scale-up using bioreactors and production in compliance with current good laboratory and good manufacturing practices.
2. Hire personnel to support clinical trial team members. This will enable them to expedite the arduous tasks related to regulatory affairs in obtaining both institutional and federal approvals for the immune-based trials. Achieving regulatory approvals is currently a rate-limiting step.
3. Undertake proof-of-principal trials initially infusing NK cells in patients with advanced medulloblastoma.
4. Expand the immunotherapy program to encompass other types of pediatric brain tumors.
THE IMPACT OF PHILANTHROPY
Dr. Cooper stands ready to implement the Noah protocol,”his investigational approach to brain cancer immunotherapy which administers propagated NK cells to children with advanced medulloblastoma. To move forward, he requires private funding to support the additional discovery and preclinical research needed to begin enrolling patients in Phase I and subsequent clinical trials. Per his previous track record, Dr. Cooper will leverage the philanthropic support he receives to obtain significant federal grants for advancing this and other immune-based therapies.
Dr. Cooper’s ongoing efforts to develop NK- and T-cell therapies stems from a desire to change the world’s approach to pediatric cancer therapy. Young cancer patients traditionally have had few effective treatment options, with their only hope tied to a “hand-me-down” approach that takes medicines developed for adults and applies them in children without conducting sufficient formal studies. However, cancer therapy invented to date for adults anticipates only a five-year survival from disease. Young patients are expected to survive for more than five years, and most of these decades-old therapies are toxic to growing children. In fact, a staggering 40 percent of young patients who beat brain and other cancers today will be significantly handicapped in the years to come as a direct result of these therapies. Thus there is no question that research specific to childhood cancers and new, safer treatments are required. Programs driven by individual patient need rather than by potential market share are critical—and viable at MD Anderson Children’s Cancer Hospital where every child with cancer is a priority, no matter the rarity of his or her diagnosis.
Donors can help Dr. Cooper and his team apply targeted NK- and T-cell therapies to patients and avoid the bottle-neck and inefficiency of the hand-me-down approach. This is particularly important at MD Anderson, as children referred here often have relapsed or metastatic cancers that are resistant to conventional therapies. But young patients everywhere else will also benefit through shared innovation.
By controlling the research, the manufacturing pipeline, and the trials, Dr. Cooper will advance new immune-based therapies that increase children’s (and adult’s) chances of surviving brain and other aggressive cancers and improve the quality of survivorship—by delivering the most effective therapy with the fewest side effects. Overall, his groundbreaking program is poised to make the first significant difference in pediatric cancer treatment in more than two decades. And philanthropic support for his work will have vital impact: there is no greater gift to young cancer patients than the chance to grow up and lead normal lives.